Abstract
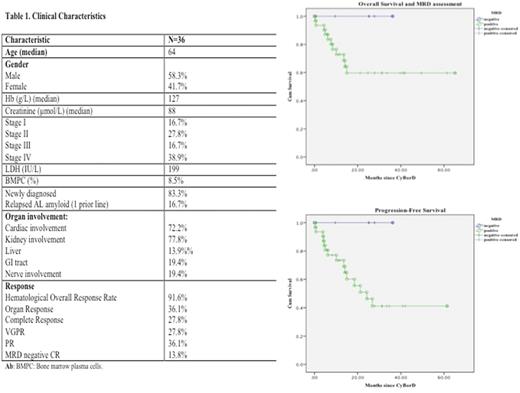
Introduction In the field of multiple myeloma (MM), the clinical utility of minimal residual disease (MRD) has been an area of extensive research. The use of MRD assessment in AL amyloid patients is an emerging area of interest. In the present study, we aimed to describe the rate of MRD negativity for patients treated with CyBorD at our Institution. Methods All consecutive AL amyloid patients treated with CyBorD at Tom Baker Cancer Center from 01/13 to 04/17 were evaluated. MRD was assessed by multiparameter flow cytometry as per Euroflow consensus guidelines in patients with confirmed complete response. A p value of <0.05 was considered significant. Survival curves were constructed according to the Kaplan-Meier method and compared using the log rank test. Results 41 consecutive patients with newly diagnosed (n=35) and relapsed AL (n=6) who had received CyBorD at our Institution over the defined period were evaluated. 5 patients were excluded due to lack of follow-up or enrolment in clinical trials. Clinical characteristics are shown in Table 1 for the remaining cases (n=36). CyBorD was administered with bortezomib at 1.3-1.5 mg/m2 SC or IV for 3-4 weeks on a weekly basis in 34 cases and biweekly in 2 patients, cyclophosphamide was given at 300 mg/m2 on days 1, 8, 15 and 22 and dexamethasone at 20-40 mg/PO on days 1, 8, 15 and 22. After a median of 5 cycles, ORR was seen in 91.6% of cases with a PR of 36.1%, VGPR of 27.8% and CR of 27.8%. MRD negativity was observed in 5/10 cases with complete response. MRD negativity rate of 13.8% was reported for the whole group of patients. Organ and cardiac responses were observed in 36.1% and 27.3%, respectively. Median OS was similar between MRD positive and negative cases (NR vs NR, p=0.1) (Fig 1a). However, none of the MRD negative cases has died or progressed. A trend towards better PFS was observed in the MRD negative cases (p=0.06) (Fig1b). In conclusion, MRD negativity was observed in ~15% cases of AL patients treated with CyBorD after a median of 5 cycles. Further studies to assess the impact of MRD negativity on clinical outcomes and its role for guided therapy are required.
Jimenez-Zepeda: Takeda: Honoraria; Janssen: Honoraria; Celgene: Honoraria; Amgen: Honoraria. Neri: Celgene: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Research Funding. Bahlis: Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal